

Whitens up to 10 shades in 7 days

Causes no significant gum irritation

Causes no significant tooth sensitivity

100% of participants saw whitening results after use
We enrolled 27 qualified men and women aging from 18 to 75. All major races were represented in the study groups. All subjects had healthy, natural maxillary teeth. Only one subject had a single crown or veneer, and some others had single aesthetic tooth colored restorations.
27 subjects were enrolled because we pre-determined that this number caused our power calculation for the study to be greater than 95%. This number of subjects also allowed us to have sufficient data to detect statistical differences in mean tooth shades of ± 0.50 within a high degree of confidence of the data being accurate.
In order to avoid influencing users’ perception during the trial, the 27 subjects were given the products in white paper bags with the manufacturer's instructions for use. The study followed a blind research protocol that complied with industry and ADA standards.
Tooth color measurement (using Vita Easyshade)
The Vita Easyshade spectrophotometer is a handheld probe device widely used for determining tooth shade color. The machine readouts were measured in triplet and the mean was taken for each subject visit. Conversion of Vita units to mathematical scores we performed are shown below:

Oral hard and soft tissue evaluation
Presence of blanching, irritation, erythema, desquamation or ulceration of soft tissues and gross changes in teeth or restorations were documented if present.
Löe and Silness Gingival Index
The Löe and Silness Gingival Index is an assessment of periodontal health that evaluates the severity of gingivitis on the basis of color, consistency, and bleeding on probing (BOP). Six gingival areas on each of the anterior maxillary teeth were scored from 0 to 3, and an average score for each participant was derived by dividing the sum of individual scores by the number of evaluable sites. Scoring criteria was as follows:
0 = normal gingiva
1 = mild inflammation (slight color changes and edema; no BOP);
2 = moderate inflammation (redness, edema, and glazing; BOP);
3 = severe inflammation (marked redness, edema, ulceration; tendency toward spontaneous bleeding);
8 = ungradable site;
9 = missing tooth.
Visual analog scale (VAS) dentinal hypersensitivity score
Subjects were asked to self-assess sensitivity by recording their perceived sensitivity on each of the six maxillary teeth using a scale of 0-10 with the pain defined as:
0 = No pain or barely noticeable tooth sensitivity
1-3 = Mild pain
4-6 = Moderate pain
7-9 = Severe pain, not constant
10 = The most excruciating pain, constant
Assessment for adverse reactions
Subjects were asked to report any adverse reactions to the whitening treatment.
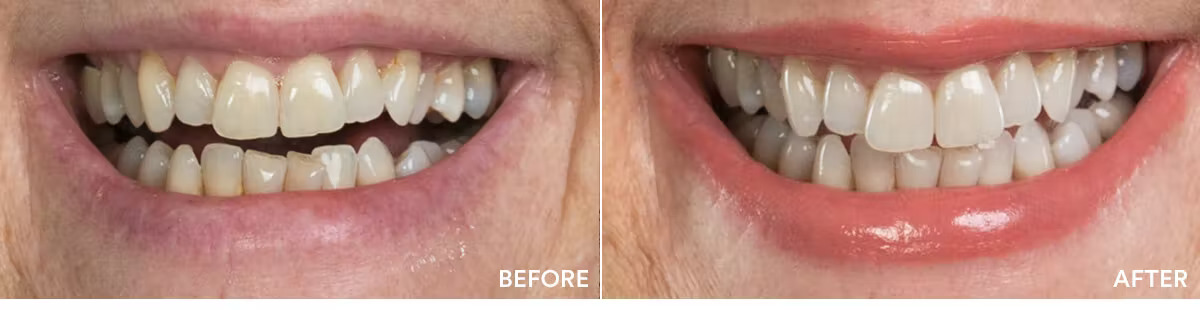
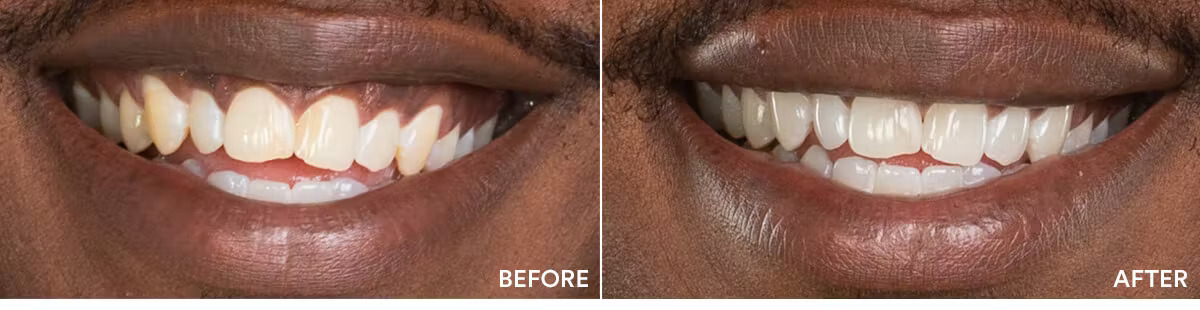
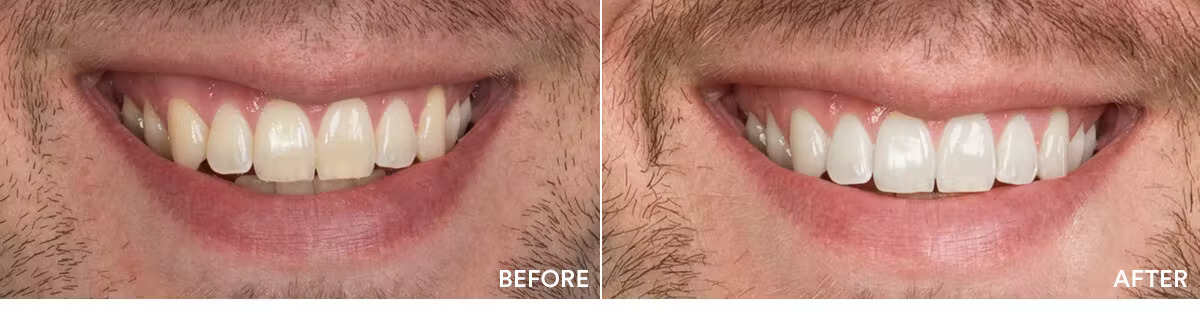
The teeth whitening results were statistically highly significant. The average shade improvement over the course of 7 days was 7.11 shades. It should be noted that 2 people or more achieved a whitening improvement of 10 shades or better.
Average shade improvement after 7 days: 7.11 shades
After 7 days, whitens up to: 10 shades
We judged safety of the Auraglow 35% carbamide peroxide whitening gel product based on the following standards.
Oral hard and soft tissue evaluation
We thoroughly monitored the hard and soft tissue of the 27 subjects entered in the study. There were no tissue changes found except for very mild reversible blanching / bleaching of the marginal gingival in about 5% of the subjects studied.
Löe and Silness Gingival Index
There was no significant gum irritation after use. The gum measurements did not change because of exposure to the Auraglow 35% carbamide peroxide gel, and that means it caused no significant gum irritation.
Visual analog scale (VAS) dentinal hypersensitivity score
Dentinal sensitivity never increased more than a half-unit, and that means that there was no significant teeth sensitivity after using the product.
Assessment for adverse reactions
In all subjects tested there were no ill effects or adverse reactions reported by the use of the Auraglow 35% carbamide peroxide gel.
In conclusion, this clinical study of the Auraglow 35% carbamide peroxide whitening gel found the following:
Each participant submitted a self-assessment questionnaire to report their own perceived use and experience with the product.